Introduction
Appropriate analytical methods are needed for toxicological and epidemiological studies as well as to enforce any restrictions. As alluded to in my article on PFAS structure, there is a not single definition for PFAS. In fact from this 19 July 2022 federal register notice on the 5th Contaminant Candidate List and this proposed rule under section 8(a)(7) of the Toxic Substances Control Act it becomes clear that the EPA lacks a single PFAS definition within itself. The EPA definitions are also different (and narrower) than the new July 2021 OECD report “Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances.” Many PFAS definitions are intentionally purpose written making one analytical method impossible to achieve. Additionally, there are many different PFAS types; the most commonly known ones are the anionic PFAS but there are also cationic and zwitterionic (both positive and negative functional groups). Given these two difficulties an immediate question should be how we measure these compounds. This article intends to cover the broad strategies taken to measure PFAS and some of the methods within each as well as the methods’ strengths and weaknesses. It focuses on anionic non-polymeric PFAS. It may be helpful to review the article on PFAS nomenclature.
Summary
In general, there are three broad strategies for PFAS analysis:
1. Target analysis
2. Total fluorine (organic fluorine) methods
3. Non-target analysis including suspect screening and the total oxidizable precursor (TOP) assay (TOPA)
PFAS analysis is severely complicated by the prevalence of fluorochemicals in laboratory equipment particularly PTFE. For example, PFAS is widely prevalent in HPLC solvent inlet tubing and PFAS from felt tip permanent markers used to mark containers during sampling campaigns.
Target analysis summary
Target analysis targeted methods typically involve either liquid or gas chromatography (LC or GC) coupled with mass spectrometry (MS) and require a reference standard for quantification. Standards are only available for select PFAS and only these specific substances can be quantified. Target analysis yields accurate concentrations but does not provide the total PFAS load.
Total fluorine methods summary
Fluorine is a sum of its inorganic and organic constituents; this insight forms the basis for quantification attempts by total fluorine methods. As PFAS are organic molecules, inorganic fluorine is typically removed from the analysis and absorbable or extractable organic fluorine is measured. Due to differing chemical properties among PFAS, the extraction method chosen will not be suitable for all PFAS classes. Absorbable organic fluorine relies on choosing a suitable absorbent so suffers from the same difficulties.
Non-target analysis summary
Non-target analysis uses chromatography coupled to a high-resolution mass spectrometer and can identify previously unknown PFAS. These methods tend to rely on quadrupole time-of-flight and linear ion trap-orbitraps which are not common instruments. If reference standards are available, similar detection limits to targeted analysis can be achieved. The TOPA deserves special mention as this method performs targeted analysis then attempts to oxidize all precursor compounds into compound endpoints which have standards then performs targeted analysis again to see the difference and theoretically quantify the precursor load but not the specific precursors. These methods suffer from the high technical capacity and equipment required to employ them.
Targeted Analysis
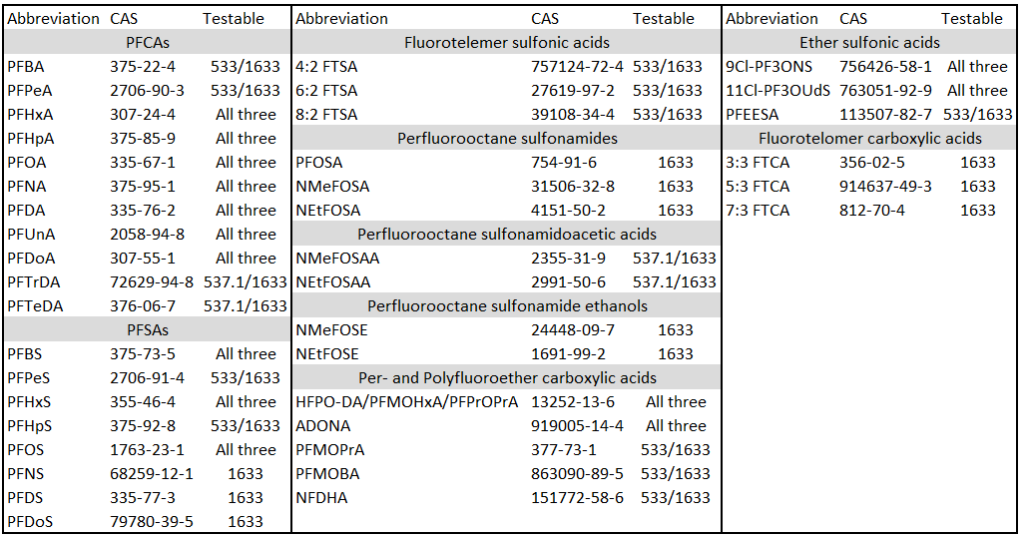
Targeted analysis is currently the only form of analysis which can support drinking water regulatory action because it is able to quantify extremely low PFAS levels; generally around 4 parts per trillion (ppt). In general, LC-MS/MS or GC-MS/MS is used. Reference standards are only available for select PFAS so only select PFAS may be quantified. Which PFAS have standards is driven both by the ability to synthesize extremely high purity samples, regulatory emphasis, and economic viability for the producers. The main PFAS which have standards are per- and polyfluorinated acids (PFAA) – particularly the carboxylic (PFCA) and sulfonic acids (PFSA) as well as the etherified versions of these. As a quick reminder PFCA end in “O” such as PFOA and PFSA end in “S” such as PFOS. The etherified versions tend to have a less standardized nomenclature structure such as “GenX chemicals” or ADONA.
Targeted analysis is well established for various matrices. For example, USEPA Methods 537.1 and 533 together cover 29 PFAS (537.1 focuses mainly on long chain PFAS and covers 18 while 533 was developed mainly to cover some more short chained PFAS and covers 25 PFAS; there are 14 PFAS which overlap between the standards). The German Institute for Standardization (DIN) has also published standard methods DIN 38407-42 and 38414-14. DINs are not freely available like the American methods so I will not link to a store here however, the DINs are broadly similar to the American methods. All these methods use solid phase extraction (SPE) followed by liquid chromatography tandem mass spectrometry (LC-MS/MS). As these methods rely on solid phase extraction, the method efficacy declines with chain length. In other words, the quantification and detection limits for short chained PFAS are higher than they are for long chained PFAS.
As mentioned in the nomenclature article, PFAS may come in “linear” or “branched” forms. These targeted methods may detect branched forms however, branched standards are not widely available and ignoring these will result in a low bias. Linear to branched isomer ratios can also help to identify the PFAS sources. The USEPA Methods 537.1 and 533 include technical notes for dealing with branched isomers.
The DINs note that these methods can be extended to other PFAA with appropriate standard availability however, volatile fluorotelomers (like fluorotelomer alcohols) cannot be determined using these methods.
Total fluorine methods
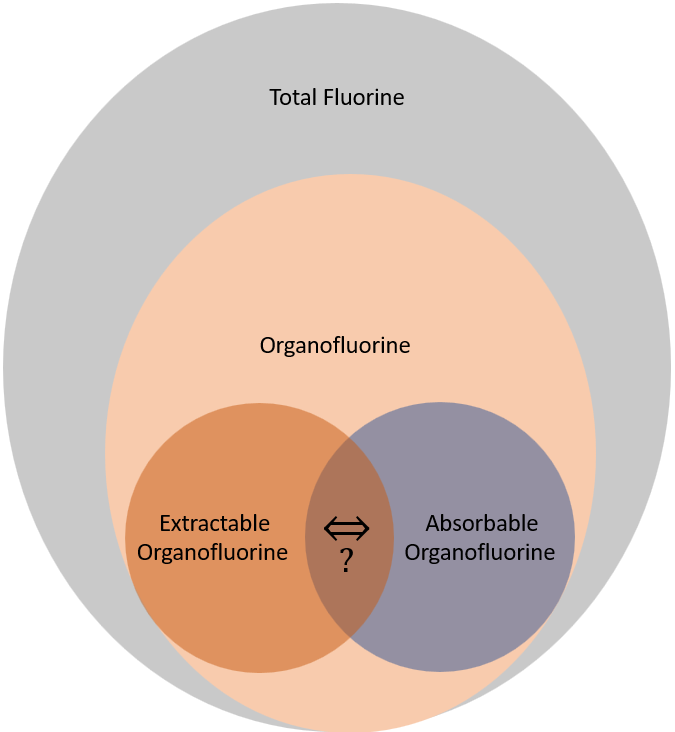
Total fluorine (TF) is the sum of Inorganic Fluorine (IF) and Organic Fluorine (OF) sometimes called Total Organic Fluorine (TOF). TOF includes fluorine content of all PFAS and their precursors in the sample. TOF methods cannot however separate out organic fluorine contained in medications or other common uses from PFAS. There have been exceedingly few studies quantifying TOF fractions from PFAS compared to medications or other uses of organofluorine. Additionally, TOF methods have much higher quantification limits than targeted analysis, typically around 100 ppt. These two limitations make TOF methods suitable for screening but not protective enough of public health to seriously be used in drinking water regulation. Remember the interim health advisories (HAs) for the apical effects of PFOA and PFOS were 0.004 and 0.02 ppt respectively, so 100 ppt is 4 or 5 orders of magnitude larger than the level at which no apical effects are expected. Additionally, organic fluorine methods only measure the fluorine while the HAs measure the full molecular weight. For example, PFOA (C8HF15O2) has a molar mass of 414.07 g/mol but the mass of fluorine only weighs approximately 285 g/mol PFOA meaning that only about 69% of each PFOA molecule would be quantified assuming perfect extraction and quantification. The exact ratio of fluorine to the overall molecular weight is species dependent.
Organofluorine analysis requires the organic fluorine to be extracted – Extractable Organic Fluorine (EOF). There will always be some organofluorine which cannot be extracted and some which can be extracted but cannot be quantified. Extraction can be the same as for targeted methods. However, due to the large variability between the PFAS chemical composition, the extraction method will influence the test results. Likewise, similar to targeted methods, there is worse extraction efficiency for shorter chained PFAS and specific substances such as trifluoroacetic acid. A special EOF method is known as absorbable organic fluorine (AOF). During AOF, the sample is typically absorbed to a mixed-mode weak anion exchange SPE; again similar to targeted analysis methods.
The most common total fluorine methods are: combustion ion chromatography (CIC), particle-induced gamma-ray emission spectroscopy (PIGE), instrumental neutron activation analysis (INAA), X-ray photoelectron spectroscopy (XPS), and 19F nuclear magnetic resonance spectroscopy (19F NMR). Other methods are under development including: inductively coupled plasma mass-spectrometry (ICP-MS), continuum source molecular absorption spectrometry (CS-MAS), defluorination with sodium biphenyl (SBP), potentiometric and fluorimetric detection, and reversed phase LC-UV or GC coupled with a flame ionization detector (FID), electron capture detector (ECD), or MS. CIC is currently the most discussed TF method.
Non-targeted Analysis
Non-target analysis for PFAS typically uses chromatography coupled to high-resolution mass spectrometers to identify previously unknown PFAS. These methods tend to rely on quadrupole time-of-flight and linear ion trap-orbitraps observed accurate masses, isotopic fingerprints, and MS/MS fragments. Accurate masses, isotope patterns, and molecular feature fragmentation patterns obtained from high resolution mass spectroscopy (HRMS) are compared to databases with known PFAS, such as the USEPA CompTox Chemistry Dashboard, NORMAN Suspect List Exchange, MetFrag, and STOFF-IDENT. Measured chemical responses are compared across two or more sample groups in a process known as fold-change comparison.
Total Oxidizable Precursor Assay
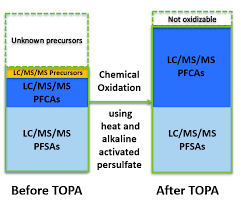
The Total Oxidizable Precursor (TOP) Assay (TOPA) was developed by Houtz and Sedlak in 2012 to quantify concentrations of difficult-to-measure and unidentified precursors of PFCA and PFSA in urban runoff. Essentially a sample is measured following conventional targeted analysis methods and then samples are exposed to hydroxyl radicals generated by persulfate thermolysis under basic pH conditions and measured following conventional targeted analysis methods again. Comparing pre- and post-oxidation concentrations the PFAA precursor load can be estimated. Unfortunately, oxidation conversion yields are compound dependent and many factors affect the oxidation process. Oxidation completeness can be tracked by adding a 13C mass labelled precursor however, this again is dependent on the specific compound chosen. Additionally, the endpoint compounds selected for targeted analysis affect the results dramatically. Activated persulfate does not generally oxidize PFSA so this can only really provide inference on PFCA precursors. The TOP assay is also labor intensive, requiring twice the number of samples and more than twice the amount of work to produce a single result. The TOP assay does provide an indication of PFAS precursor load without requiring as advanced equipment or training as non-targeted analysis.
Conclusions
Each strategy selected for PFAS analysis carries with it its own unique strengths and drawbacks. Under most current toxicological frameworks employed by regulatory agencies targeted analysis is the only real viable option however, this can miss many PFAS. Total fluorine methods are several orders of magnitude too imprecise to accurately protect public health but capture most PFAS. Non-targeted analysis methods require expensive equipment, much experience, as well as being non-quantified.
Further Information
This article is one of several on PFAS. You can read about PFAS discovery, structure, nomenclature, or my other PFAS articles.
- Benchmarking and Publications for Non-Targeted Analysis working group (BP4NTA)
- TSCA Chemical Substance Inventory
- USEPA Substance Registry Services (SRS)
- NORMAN Suspect List Exchange
- Houtz and Sedlak “Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff”
- USEPA Method 537.1
- USEPA Method 533
2 thoughts on “PFAS Analytic Strategies”