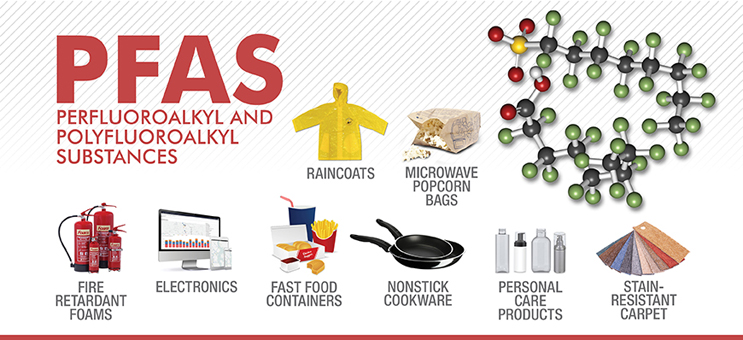
Per- and polyfluoroalkyl substances (PFAS) are a ubiquitous emerging public health threat. PFAS terminology can be extremely confusing because it looks like an alphabet soup of similar acronyms. This article is the first of two on PFAS terminology. This one intends to provide an overview of PFAS structure, the second one intends to help demystify PFAS terminology and nomenclature. My previous article on PFAS was on Dr. Roy Plunkett’s discovery of PTFE, a tetrafluoroethylene fluoropolymer.
Scope
The EPA’s comptox database lists over 9,252 PFAS; this is too large a class to name on individual bases. A systematic naming convention has evolved so that much information is conveyed with just the name. There are 5 main ways to name fluorinated compounds: the perfluoro nomenclature, F-nomenclature, code numbers, the International Union for Pure and Applied Chemistry (IUPAC) nomenclature, and Chemical Abstract Service (CAS) nomenclature systems. Additionally, fluorine chemistry as a field has existed long before those five systems were created and naming was not really standardized until Buck et al tried to harmonize conventions in 2011. The idea of this article is to provide an overview of common PFAS groupings, the second article will go into the five naming conventions.
Introduction
PFAS is a general nonspecific name encompassing a group of substances. There is no clear agreed upon definition of what exactly counts as PFAS. The Organization of Economically Developed Countries (OECD), the United States Environmental Protection Agency (EPA), and the World Health Organization (WHO) have all used various definitions. In the paper meant to harmonize conventions, Buck defined PFAS as “highly fluorinated aliphatic substances that contain 1 or more carbon atoms on which all the hydrogen substituents (present in the nonfluorinated analogues from which they are notionally derived) have been replaced by F atoms, in such a manner that they contain the perfluoroalkyl moiety CnF2n+1–.” This definition was the first clear PFAS definition. A moiety means part of a molecule. The OECD/United Nations Environment Program (UNEP) pointed out that Buck’s definition left out many substances commonly recognized as PFAS such as perfluorodicarboxylic acids and recommended the –CnF2n– moiety instead, although there is still debate surrounding this. Buck’s definition was updated by Glüge et al and informed the OECD/UNEP definition. Glüge’s definition expands Buck’s to cover perfluorocarbon chains with functional groups on both ends, aromatic substances with perfluoroalkyl moieties on side chains, and fluorinated cycloaliphatic substances.
Historical Note
As mentioned in the PFAS discovery article, PFAS were initially investigated to replace sulfur dioxide, methyl chloride, ammonia, and other extremely dangerous early refrigerants. This may jog your memory; the first major public PFAS concerns were mainly focused on perfluorocarbons under the 1987 Montreal and 1997 Kyoto Protocols. The Montreal Protocol aimed to limit chlorofluorocarbons. The Kyoto Protocol aimed to limit 7 greenhouse gas (GHG) categories and stated that climate change is real and driven by anthropogenic emissions. The GHG categories included perfluorocarbons (PFCs) and hydrofluorocarbons (HFCs).
Unfortunately, early 2000 environmental scientists adopted the PFC acronym for PFAS instead of the perfluorocarbons identified in the Kyoto Protocol. There is still quite some confusion caused by this. While PFC is used in turn of the millennium PFAS literature it should be avoided now.
General Organofluorine Concepts
Carbon (C) bonded to fluorine (F) is the class basis for PFAS. The C-F bond is extremely persistent and the key factor for the problems PFAS causes as well as its desirable technical properties. Carbon can form up to 4 bonds with other atoms. The first broad distinction is between polymers and non-polymers. A polymer is a molecule made up of repeating subunits called monomers. Polymers often serve as the feedstock to produce other PFAS chemicals, the most widely recognized fluoropolymer is polytetrafluoroethylene (PTFE) which Dr. Roy Plunkett discovered on 6 April 1938.
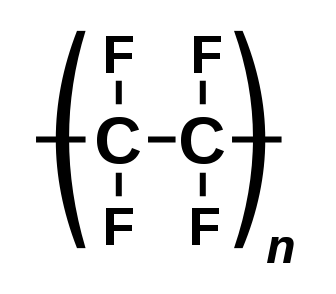
Functional groups often serve as the basis for classification systems. A functional group is a moiety made of a specific structure which causes characteristic chemical reactions which are similar no matter what the rest of the molecular composition is. PFAS chemicals can also exist in multiple states such as acids, anions, cations, and salts which have important implications for their physical and chemical properties. Anionic forms are the environmentally prevalent form and the general form most authors refer to. Some legislation, particularly European Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH), defines substance to include impurities and stabilizers of the main PFAS compound.
Broad non-polymeric structure
Non-polymeric PFAS include perfluorinated and polyfluorinated substances. When carbon is bound only to fluorine except for functional groups it is called perfluorinated and the compound is saturated with F. When some carbon bonds include things other than functional groups or fluorine it is called polyfluorinated and is considered unsaturated with respect to F; hydrogen is the most common other bond. As PFAS stands for “per- and polyfluoroalkyl substances” and no single compound can be both unsaturated and saturated with respect to fluorine simultaneously “PFAS” is by default a plural acronym. Both per- and poly-fluoroalkyl substances are subdivided into classes where the functional groups behave relatively similarly. Two large classes are fluoroalkyl acids and fluoroalkane sulfonamides. These large classes are then subdivided again based on functional groups/moieties. For example, perfluoroalkyl acids are further subdivided into carboxylic, sulfonic, sulfinic, phosphoric, phosphinic, and other acid types. There are about 425 common moiety-based subdivisions within PFAS.
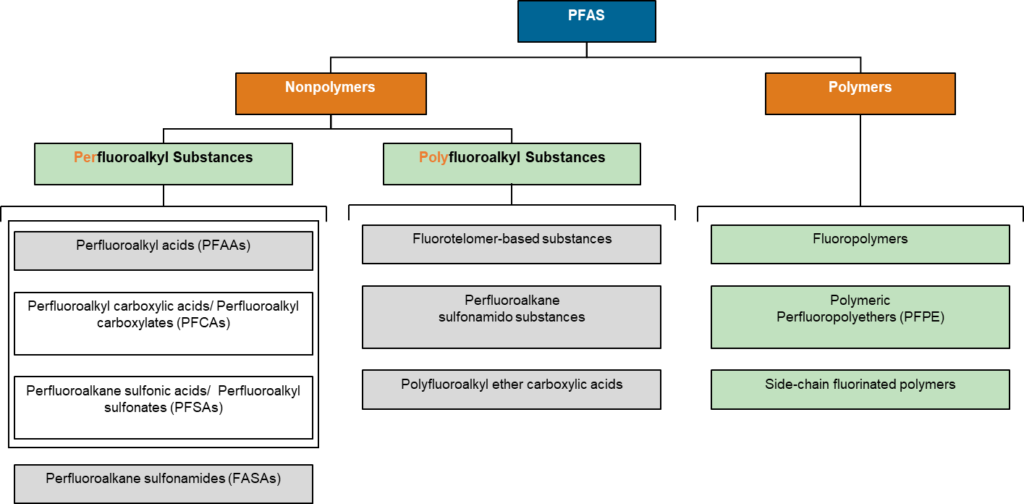
In 2017, Wang et al published an iconic chart covering the number of papers published on various PFAS groups reproduced below.
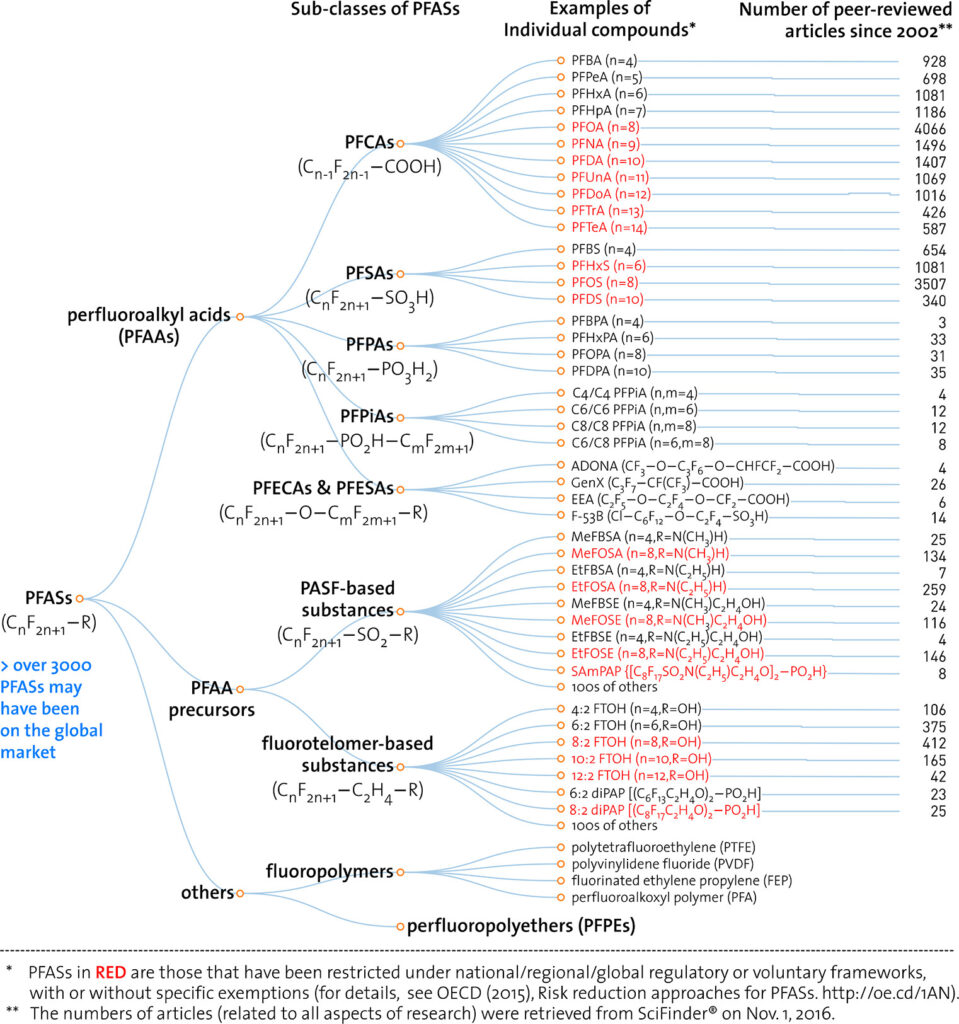
Conclusion and Key Take Aways
There is no clear definition on what PFAS encompass, even within regulatory organizations. There are about 9,252 PFAS substances which are broadly divided into polymeric and non-polymeric substances. Non-polymeric PFAS subclasses are based on carbon chain saturation with fluorine into full saturated (perfluoroalkyl) or non-fully saturated (polyfluoroalkyl) groups. Those groups are subdivided based on moieties/functional groups so that compounds which behave similarly are classified together. An upcoming article will cover the five common classification systems for individual compounds.
Other articles in the series include PFAS Discovery
Thanks for laying out part of the crux of the issue…the complexity due to the quantity of possible compounds that need to be tested for, quantified, regulated, etc.